 |
||||||||||||||||
 |
||||||||||||||||
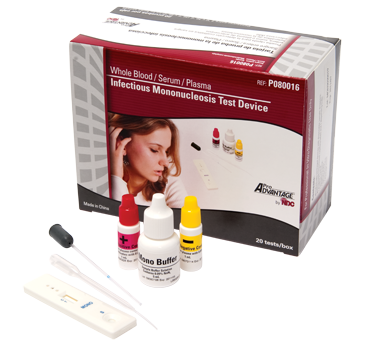 |
Infectious Mononucleosis Test DeviceA rapid chromatographic immunoassay technology identifies heterophile antibodies in whole blood, serum or plasma to aid in the diagnosis of infectious Mononucleosis. Rapid chromatographic immunoassay technology identifies heterophile antibodies in whole blood, serum or plasma to aid in the diagnosis of infectious Mononucleosis. Qualitative membrane strip based immunoassay for detection of IM heterophile antibodies. All inclusive test-kit with 20 tests. Simple to perform using whole blood (from venipucture or fingerstick), serum or plasma. Reliable results in 5 minutes. CLIA waived for whole blood and FDA 510(k) cleared to market. INFECTIOUS MONONUCLEOSIS TEST DEVICE BROCHURE (PDF) CLSI PROCEDURES (.doc)MODERATE | WAIVED
|
|||||||||||||||
| |
||||||||||||||||